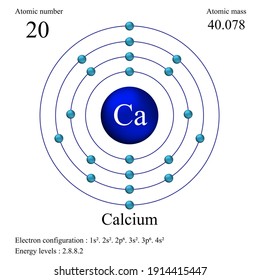
The chemical element Calcium (Ca), atomic number 20, is the fifth element and the third most abundant metal in the earth’s crust. The metal is trimorphic, harder than sodium, but softer than aluminium. A well as beryllium and aluminium, and unlike the alkaline metals, it doesn’t cause skin-burns. It is less chemically reactive. Well it depends on the symbol Top left: atomic number (number of protons) = 20 Top right: atomic mass - gives an indication of the average number of neutrons - in this case (40–20).

Ca I Ground State 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 1 S 0 Ionization energy 49305.95 cm-1 (6.11316 eV) Ref. SC85 Ca II Ground State 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 S 1 / 2 Ionization energy 95751.87 cm-1 (11.87172 eV) Ref. It is not always easy to make sensible comparisons between the elements however as some bonds are quite short because of multiple bonding (for instance the O=O distance in O 2 is short because of the the double bond connecting the two atoms. The bond length in CaCa is: 394.7pm. There are several other ways ways to define radius for atoms and ions. Google firefox sync.
Calcium Chemical Property
Molar mass of Ca = 40.078 g/mol
Convert grams Calcium to moles or moles Calcium to grams
| Symbol | # of Atoms | Calcium | Ca | 40.078 | 1 | 100.000% |


In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
Calcium Atom Mass
A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
Dsg transmission service kit. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
Calcium Atomic Wt
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
Facts About Calcium Atoms
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.